





Published on Sep 16, 2023
The objective: A high content of nitrites in foods is very harmful for us, as it could cause cancer and other severe health problems. Our project will be performing an experiment focused on the content of nitrite in different foods, as well as studying and practicing the analytical method of spectrophotometry. By doing the project, we have been able to see more technologies used in the field of science, and understood how these machines are playing an important role in the lab and affecting ordinary lives.
Summary: A high content of nitrites in foods is very harmful for us, as it could cause cancer and other severe health problems.
Our project will be performing an experiment focused on the content of nitrite in different foods, as well as studying and practicing the analytical method of spectrophotometry. By doing the project, we have been able to see more technologies used in the field of science, and understood how these machines are playing an important role in the lab and affecting ordinary lives.
During Eric's last few years in China, he heard newscasters talking about nitrite related deaths, and that this or that food contains nitrites. It would seem that nitrites exist in the food we eat everyday and that they are not so far away from our lives. So a question comes up: Because we are exposed, are we safe? With our interest in chemistry, we have also heard quite a bit about analytical chemistry and thought of looking deeper into it. So we have perfomed research on the microanalysis of nitrites and perfomed the experiment on foods that are consumed daily.
Softwares: WYSIWYG Web Builder 5.0, Microsoft Excel 2007, SoftmaxPro
Hardwares: Thinkpad L430, iPhone 4, SpectraMax Plus384 Absorbance Microplate Reader (Spectrophotometer) created by Molecular Devices®
Reduced oxygenation of hemoglobin (methemoglobinemia) has been reported after exposure to nitrite-contaminated drinking water; this is also called the “blue baby syndrome” because of the cyanotic (oxygen-deficient) symptoms that result from the reduced oxygenation of the blood. Infants less than 4 months old are the most sensitive population for methemoglobinemia following exposure to nitrates and nitrites, but it does occur in older age groups. Severe methemoglobinemia can lead to coma or death. The U.S. EPA concluded that there was conflicting evidence in the literature as to whether exposures to nitrates or nitrites are associated with cancer in adults and in children. The types of cancers studied included non-Hodgkin’s lymphoma as well as stomach and gastric cancers in adults; and brain tumors, leukemia, and nasopharyngeal cancers in children.
January 14, 2014, a girl at a university in Guangxi, China, dropped sodium nitrites into the water machine in her dorm accidently when changing the water, and two of her roommates were killed.
During Chinese New Year in Yangzhou, China, a girl soon found bruising all over her body after having marinated meat with two other people. They all went to the hospital. The two other people left the hospital after two days, leaving the girl in the hospital for two more days. It is said that the nitrites in the meat caused this.
December 18, 2013, an old man accidently used sodium nitrite as salt when cooking, causing two boys' death; the man was sent to the ICU and recovered later.
December 2013, a store manager in Xuzhou, China sold sodium nitrite solution as white spirit, causing the death of two workers.
1. Step 1 Assay Buffer - ready to use from kit. Warm to room temperature for nitrate tests. If desired, the assay buffer may be more quickly warmed in a 30ºC water bath.
2. Step 2 Prepare 3 N HCI by adding 4 ml concentrated HCI to 12 ml d-I water. Mix.
3. Step 3 Add 15 ml 3 N HCI to Color Reagent No. 1 bottle. Mix by shaking well.
4. Step 4 Add 15 ml d-I water to Color Reagent No. 2 bottle. Mix by shaking well.
5. Step 5 Remove tube of NADH from amber bag, tap tube to settle contents, add 1.5 ml d-I water and replace cap. Mix by inversion several times. Keep on ice during use.
6. Step 6 Remove NaR vial from foil pouch and tap tube to settle contents before opening. Twist off the end of the Enzyme Diluent Squeeze Bulb and completely empty the contents into the NaR vial. Replace the cap and mix by inversion 3 times. Allow to stand at room temperature for at least 10 minutes, with mixing at 5 and 10 minutes. Then keep on ice during use.
Transfer 1 ml of 100 ppm Nitrate-N Standard into a test tube containing 9 ml d-I water to make a 10 ppm Nitrate-N Standard. Use the 6 microtubes to prepare Nitrate Standards as shown in table on the next page. Cap and mix the tubes by inversion before use.
1. STEP 1 Pipette 50 µl d-I water into one test tube for use as reagent blank.
2. STEP 2 Pipette 50 µl of the samples and standards into the required number of test tubes.
3. STEP 3 Add 900 µl Assay Buffer to each tube.
4. STEP 4 Add 50 µl NADH solution to each standard tube. Mix thoroughly with a vortex-type mixer.
5. STEP 5 Add 20 µl NaR solution to each standard tube. Mix thoroughly with a vortex-type mixer.
6. STEP 6 Let standard tubes sit for 20 minutes at room temperature.
7. STEP 7 Add 500 µl Color Reagent No. 1 to each tube. Mix thoroughly with a vortex-type mixer.
8. STEP 8 Add 500 µl Color Reagent No. 2 to each tube. Mix thoroughly with a vortex- type mixer.
9. STEP 9 Let tubes Stand at room temperature for 10 minutes. To ensure homogeneous samples, briefly mix the tubes with a vortex-type mixer.
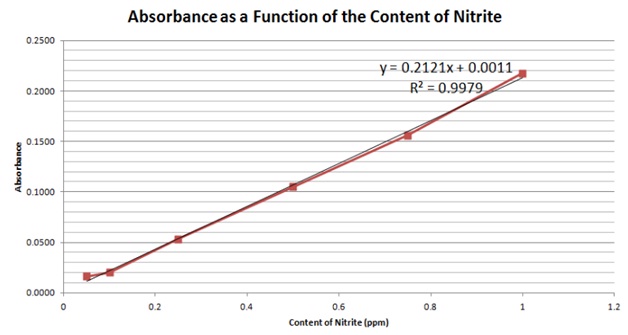
10. STEP 10 Read absorbance at 540 nm ± 20 nm in colorimeter or spectrophotometer for the samples and Nitrate Standards.
11. Repeat this procedure for each food sample tested, and compare results.
As samples themselves, the yogurt and meat both had a slightly red color before adding in the color reagents, which could possibly bring a higher absorbance and a higher content in the results. The milk and cheese were homogeneous solutions and had to be centrifuged, which will also cause a higher result. Also, our samples were only cut in small pieces and leached. This could cause the nitrites in samples not to be completely leached, causing us to end up with a lower result. Also, we only took approximately 1g of each sample and diluted it with 4mL of water to perform the experiment. If we were able to more accurately mesure and record the masses of the samples, we would be able to have more accurate results.
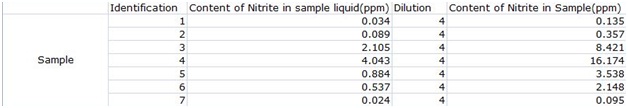
According to the results, the ham, which was a sample of processed meat, had the highest content of nitrites of about 16.174ppm, followed by the meat with 8.421ppm, then the yogurt with 3.538ppm, the cheese had 0.357ppm, the onion had 0.095ppm, the banana had 2.148ppm and the milk had 0.135ppm. The results for the ham and fresh meat corresponded to the hypothesis, showing that accumulation in the food chain does have an impact on the content of nitrites in natural foods.
The milk did not have a high content of nitrites, which may mean that the output of animals does not carry hazardous substances from the matrix, but this might also only be limited to milk products. The yogurt had a higher content of nitrites than the cheese and milk. This means that more frementation doesn't mean a higher content of nitrites as we had hypothesized. Instead, at the middle stage of frementation, the product had the highest content of nitrites. The banana had a higher nitrite content than the onion, which we believe is just a variable of the type of fruit.